The universe is a vast, marvelous and awe inspiring place, and as the great Carl Sagan once said, we humans, are a way for the cosmos to know itself. The grade nine unit of Earth and Space Science focuses on astronomy, the study of the universe and its major components. Sadly, it is hard to observe astronomical phenomena while at school, but thanks to computer programs we can bring some aspects of the cosmos into the classroom. In order to study the constellations and explore the characteristics of stars, students use a program called Stellarium on our school computers to explore the night sky, and the constellations that have captivated us for thousands of years.
Biochemical Molecules and Cells
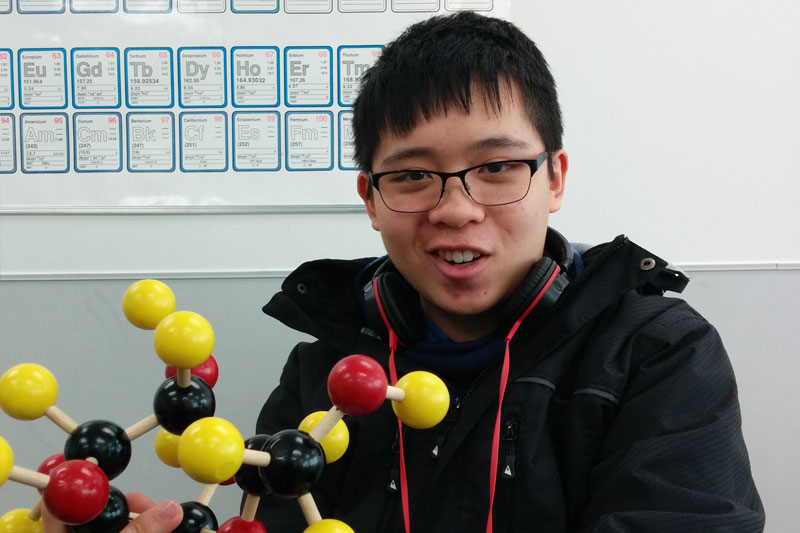
Although cells are the smallest organized units of living things, they are made of even smaller parts. These biological compounds that make up the structures of cells themselves are constructed from the macronutrients we call carbohydrates, proteins, and lipids. In order to get a more in depth understanding of these molecules, students were given molecule kits do build and observe them in three dimensions.
Elephant Toothpaste Lab
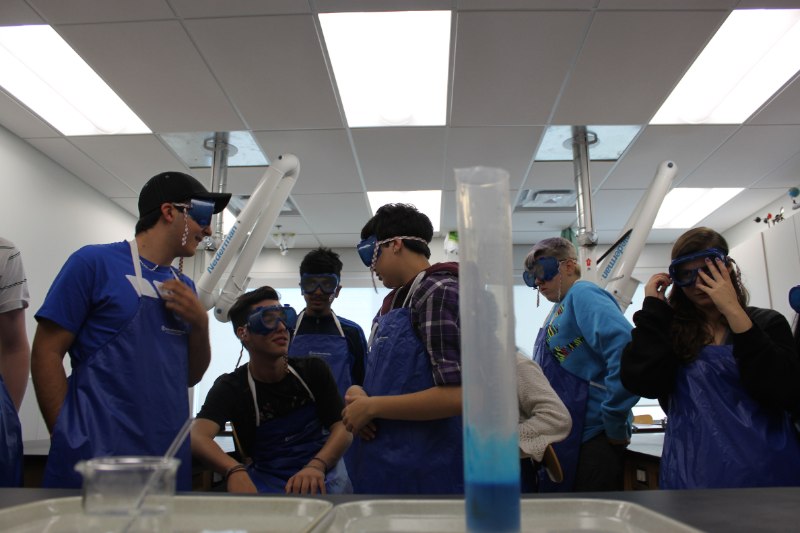
Learning about the 6 types of chemical reactions is not always exciting for everyone, especially if it is done solely through practice worksheets. Furthermore, simply discussing and being able to recognise the different types of chemical reactions can be somewhat intimidating to most students.
But life is not always about remembering equations and formulas, because let’s face it; most students forget the majority of the equations and formulas they have learned once they leave the walls of educational institutions. If, however, you can help students see through the equations and formulas and how they apply to the world they experience around them, they will remember that for many years after they have forgotten the formulas and equations.
In order to bring the chemistry from the whiteboard and worksheets to life, we look at a real-time example of a decomposition reaction. We take a mixture of hydrogen peroxide and add to it a few drops of food colouring (for effect) and a few drops of liquid dish soap (for the wow factor). In years past, we have added yeast to speed up the reaction, but this year we tried potassium iodide and a higher concentration solution of hydrogen peroxide for a bigger and better effect. Alas, as happens in science from time to time, the reaction was no different than years past, however, the what the students witnessed still evoked the usual oohs and aahs as the oxygen produced from the decomposition of hydrogen peroxide quickly expanding the soap bubbles in the solution and quickly foamed up and over the graduated cylinder.
Check out the rest the rest of the pictures on our Facebook page!
Jan 20th, 2014 – Application of Pascal’s Law
Pascal’s Law states that any pressure applied to a static (non-moving/flowing) liquid in a closed container will be transmitted undiminished to every part of the fluid, as well as to the walls of the container. This is the law/principle that explains how hydraulic car lifts work. Although the pressure is the same at all points in a static liquid, the force (depending on the area it is exerted over) does not necessarily stay the same. If, for example, some small force (F1) is applied over a small area (A1), a certain amount of pressure is produced (P1). This, in fact, is the definition of pressure P1 = F1/A1. Since the pressure at any other point (say P2) will be the same, if the area (A2) is larger than A1, the force produced will be large. This can easily be shown by re-arranging the formula P1 = F1/A1 into F2=P2xA2. Since P2 is the same as P1, but A2 is larger than A1, you can easily see that the force is increased.
Example
10 N of force is applied to an area of 5 cm2
P1 = F1/A1
P1 = 10 N/5cm2
P1 = 2 N/cm2
P2, by Pascal’s Law also equals 2 N/cm2
If that pressure is applied over a larger area (A2), say 10 cm2 we can see that the force will be 20 N
F2 = P2xA2
F2 = 2 2/cm2 x 10 cm2
F2 = 20 N (DOUBLE THE FORCE)
This can be demonstrated with a neat demo using a glass soda/beer bottle filled with water. The top of the soda/beer bottle has a small area and the bottom a larger one. If you hit the top of the bottle with the palm of your hand with sufficient force, the pressure is transferred to the water equally in all directions, and since the bottom of the bottle has a larger area, the force you hit the top with is multiplied, and it knocks out the bottom of the bottle. Science works!